Research Highlights
Teeth Illuminate Great Ape and Human Evolution
Teeth are the most abundant elements in primate fossil assemblages, are under a strong degree of genetic control, and contain the most precise developmental records of any physiological system in the body. Tooth microstructure, a primary focus of my research, is critically important for understanding development and evolution as incremental lines permanently record each day of enamel and dentine formation, remaining unchanged for millions of years. These tiny growth lines can be used to accurately determine age at death in juvenile dentitions, as well as the precise timing of childhood diet transitions, physiological stress (including birth), and environmental variation. Furthermore, dental development is correlated with primate life history, or the overall pace of growth and reproduction.
My research program encompasses aspects of paleoanthropology, oral biology, and elemental chemistry, and employs fundamental histological (microscopic) approaches as well as state-of-the-art elemental and X-ray imaging techniques. In order to augment these approaches I have assembled an international collaborative network, including experts who recover great ape and human fossil material, pioneer imaging methods, and study living great apes in the wild. Our work has led to a major revision of how we understand dental development in our closest living relative, the chimpanzee; introduced and validated powerful methods for the study of tooth growth and mineralization; and demonstrated that living and fossil Homo sapiens have a prolonged period of dental development relative to Neanderthals and earlier hominins. Ultimately, I am motivated by a desire to understand fully how teeth grow, why they vary, and how this information can advance the field of human evolutionary biology.
Foundations of Dental Tissue Structure and Development
Members of the Dental Hard Tissue Laboratory work to explore unresolved aspects of tooth biology in order to improve knowledge of our evolutionary history. In collaboration with Paul Tafforeau of the European Synchrotron Radiation Facility (Grenoble, France), we’ve conducted novel dental tissue studies with synchrotron X-ray virtual histology (Smith and Tafforeau, 2008; Tafforeau and Smith, 2008). Highlights include the first application of virtual histology to document the growth of enamel microstructure in 4D (spatially and temporally) (Tafforeau et al., 2012), which began as the honors thesis project of former Harvard College undergraduate John (JP) Zermeno ’09.
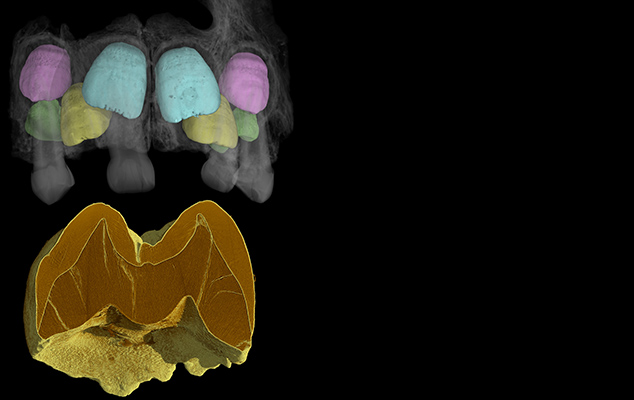
Our lab has also completed large-scale micro-CT studies of living and fossil species of Homo and Pongo, which demonstrate that enamel thickness within a genus may change over relatively short time periods, and underscore the fact that thick tooth enamel is not a defining hominin characteristic (Smith et al., 2011, 2012a,b). Additional projects within this theme include demonstrations of the taxonomic utility of internal dental structure and development in Homo erectus and Neanderthal molars (Smith et al., 2009a,b, 2012a), which are of particular significance as external dental characteristics are often unreliable for sorting mixed fossil assemblages within Asia or Europe. We also engage in functional studies of primate teeth, documenting the material properties of great ape enamel (Lee et al., 2010), and investigating the ecological correlates of orangutan and macaque enamel thickness (Smith et al., 2012b; Kato et al., 2014). These studies represent part of a growing consensus that knowledge of tooth enamel thickness alone is insufficient for predicting paleodiets in fossil apes and humans. Moreover, we have demonstrated that enamel thickness may be directly related to environmental variation in certain primates (Kato et al., 2014). On-going projects include studies of the regulation of circadian features and the timing of tooth mineralization for paleoenvironmental reconstruction.
Progress in Understanding Human Evolutionary Biology
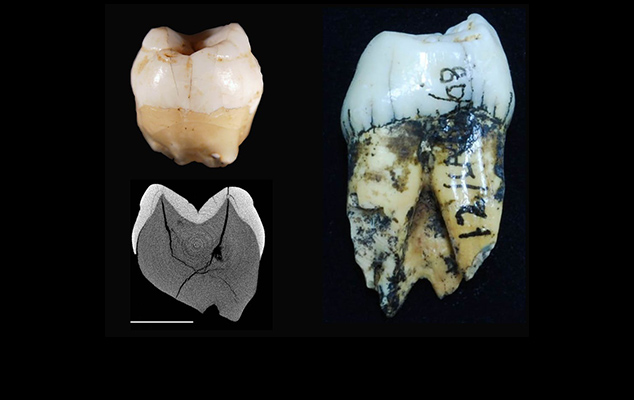
The majority of our work applies knowledge of tooth development biology for understanding hominin evolution, including the evolution of human life history (reviewed in Smith, 2013). A particularly exciting aspect of synchrotron imaging is the potential to characterize dental development non-destructively at a microscopic scale, advancing our understanding of growth processes in numerous hominin juveniles that were previously unavailable for comprehensive histological studies (Smith et al., 2007a, 2010a, 2015). Our international team of paleoanthropologists and postdoctoral fellows conducted a long-term study of Neanderthal and fossil Homo sapiens juveniles, yielding the first precise comparison of dental ontogeny in these two species (Smith et al., 2010a). We demonstrated that modern humans have a uniquely prolonged period of dental growth and development, and when considered in light of primate life history theory, these results suggest that a long childhood and late age at first reproduction may have arisen in Homo sapiens. Work on earlier hominins is revealing a surprising degree of developmental variation in australopiths and early Homo, providing critical information for age at death assessments and studies of cranial and postcranial ontogeny (Smith et al., 2015).
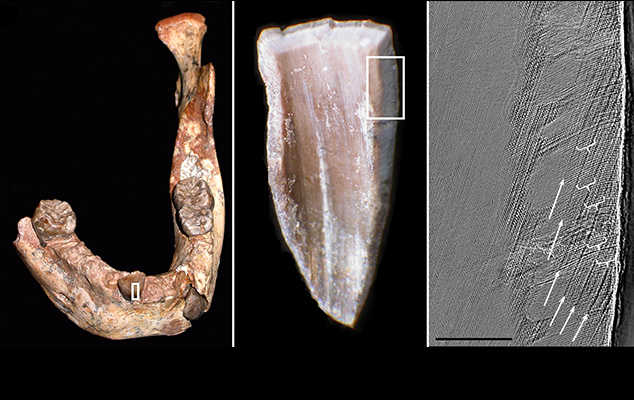
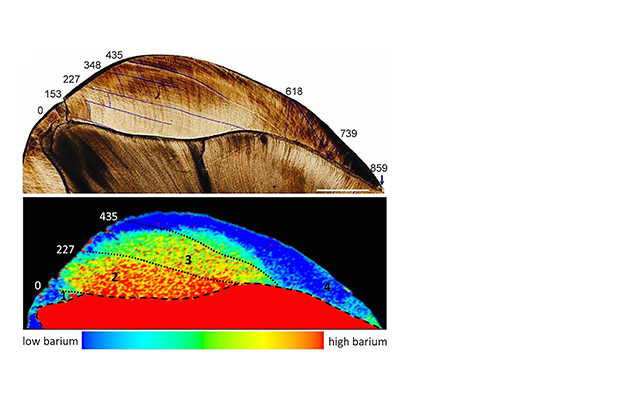
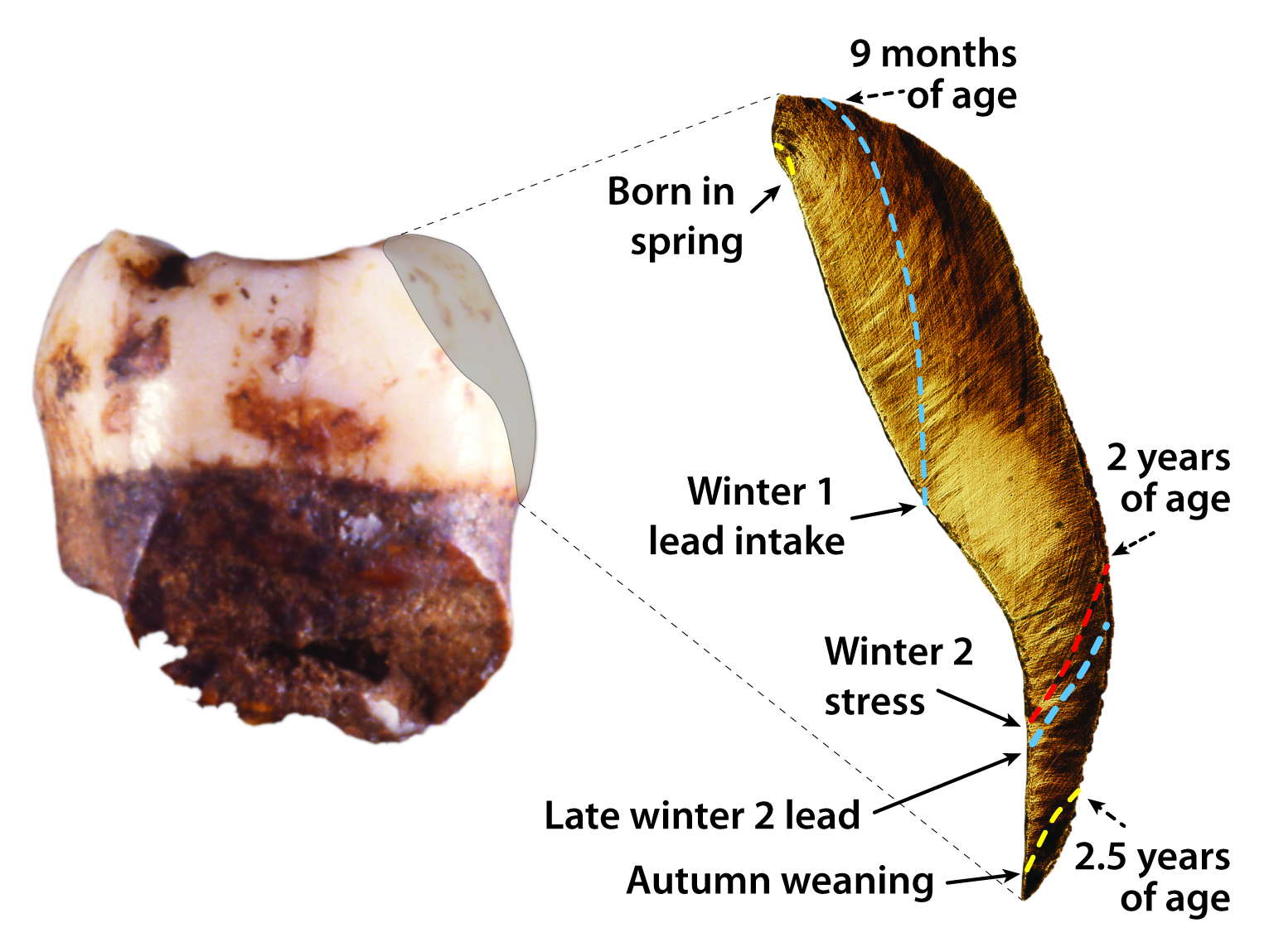
We’ve also recently proven the efficacy of a fine-scale elemental sampling approach for documenting the timing of diet transitions in human children, captive macaque infants, and wild orangutans (Austin et al., 2013, Smith et al. 2017). Barium/calcium (Ba/Ca) trace element ratios in teeth reflect barium intake via mother’s milk, which can be used in concert with growth lines to accurately age early-life diet transitions including birth, exclusive nursing, solid food supplementation, and the cessation of suckling. We applied this technique to document diet transitions over the first 2.4 years of life in a juvenile Neanderthal, which showed a period of exclusive breastfeeding for seven months, followed by an abrupt termination of maternal milk input at 1.2 years of age. This is the first precise documentation of nursing history in a fossil hominin, permitting direct knowledge of the timing of weaning, a key aspect of human life history that appears derived in comparison to our closest living ape relatives. When applied to well-preserved hominin dental remains, this approach may help to resolve ongoing debates about whether Middle and/or Upper Paleolithic Homo sapiens had a more abbreviated period of nursing than Neanderthals and earlier hominins.
Members of the Dental Hard Tissue Laboratory are currently investigating aspects of human evolution and development that have been the subject of considerable paleoanthropological debate. Harvard graduate student Kate Carter has assessed claims about the significance of external (non-metric) dental characters for reconstructing evolutionary relationships among hominin fossils, concluding that additional evidence is needed to discern how the newest hominin species Australopithecus sediba relates to australopiths and members of the genus Homo (Carter et al., 2014). We have also completed studies of molar formation and tooth eruption in captive and wild chimpanzees, demonstrating that tooth formation and eruption standards had previously been overestimated in our closest living relative (Smith et al., 2007b, 2010b). Recent work includes the first longitudinal observations of first molar (M1) emergence and feeding behavior (including nursing) in several infants (Smith et al., 2013). This collaboration with Harvard University primatologists Richard Wrangham and Zarin Machanda represents an important revision in our understanding of the relationship between tooth development and primate life history, as an influential theory posited that primate M1 emergence is coincident with the cessation of nursing, and thus could be used to predict weaning age in juvenile hominins with emergent first molars. However, we demonstrated that five wild chimpanzees continued to nurse for a year or more after their M1s emerged, and that M1 emergence in these individuals appears to relate to the development of adult feeding behaviors rather than the reproductive behavior of their mothers. On-going projects aimed at elucidating aspects of human evolution include studies of the predictive power of first molar emergence for predicting life history variables in living humans and chimpanzees, as well as studies of the relationships among dental development, life history, and phylogeny among congeneric primate species.